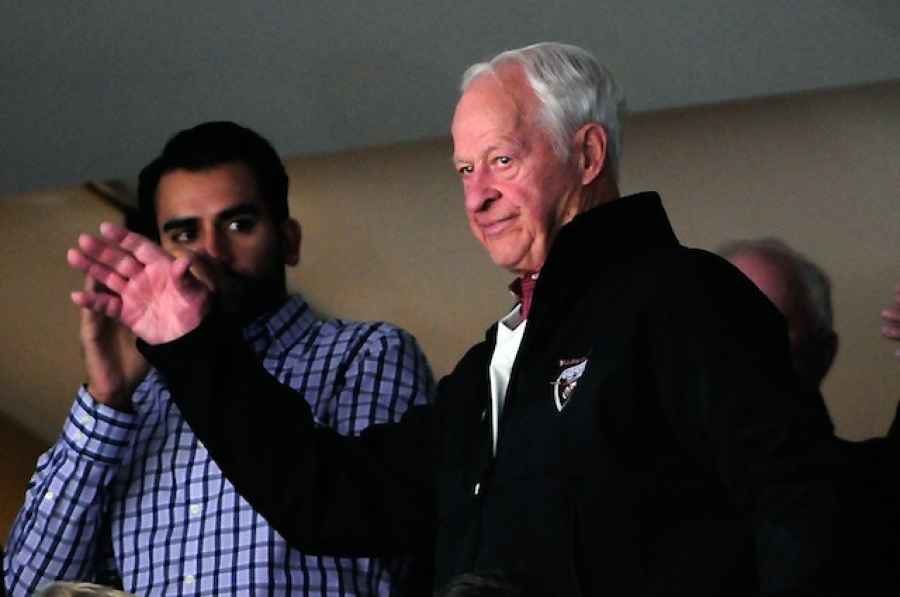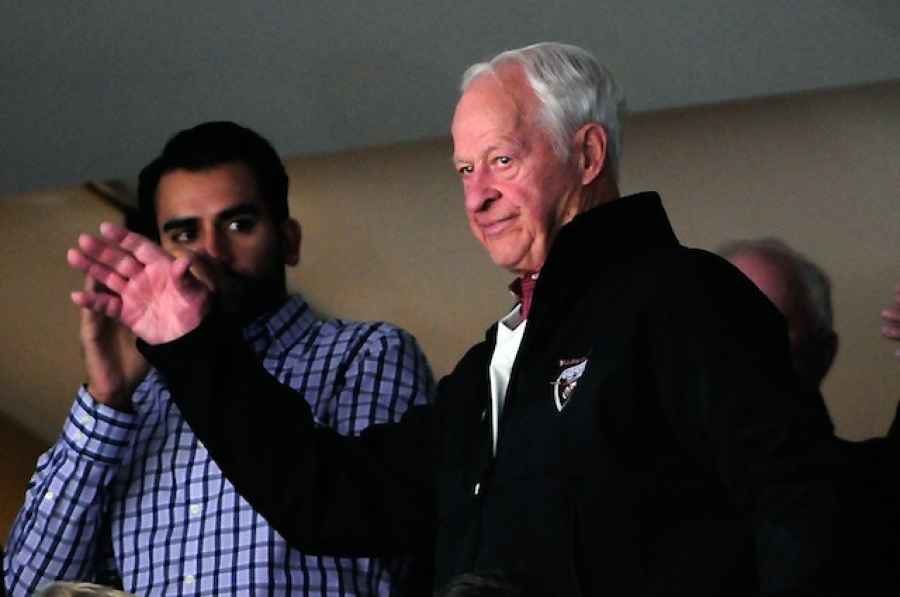) is currently conducting federally licensed and Institutional Review Board approved clinical trials for several medical conditions, including
stroke, using Stemedica's stem cell products. At the time we were contacted, Mr. Hockey had been rapidly declining and was essentially bedridden with little ability to communicate or to eat on his own.
After reviewing the information on Stemedica and Novastem, our family decided to give our father this opportunity. On December 8, Mr. Hockey underwent a two-day, non-surgical treatment at Novastem's medical facility. The treatment included neural stem cells injected into the spinal canal on Day 1 and mesenchymal stem cells by intravenous infusion on Day 2. His response was truly miraculous. At the end of Day 1 he was walking with minimal effort for the first time since his
stroke. By Day 2 he was conversing comfortably with family and staff at the clinic.
On the third day, he walked to his seat on the plane under his own power. By Day 5 he was walking unaided and taking part in helping out with daily household chores. When tested, his ability to name items has gone from less than 25 percent before the procedure to 85 percent today. His physical therapists have been astonished. Although his short-term memory, strength, endurance and coordination have plenty of room for improvement, we are hopeful that he will continue to improve in the months to come.
As a family, we are thrilled that Dad's quality of life has greatly improved, and his progress has exceeded our greatest expectations. Once again, we cannot emphasize how much you have fueled Mr. Hockey's recovery and we thank everyone for their continued prayers and support."